
What is "Hard Water" ?
Hard Water is defined as water that contains dissolved minerals (usually but not limited to calcium
and magnesium bicarbonate) expressed in grains per gallon.
The lower the mineral content of the water, the “softer” the water (and the more corrosive); while
higher mineral content means “harder” water.
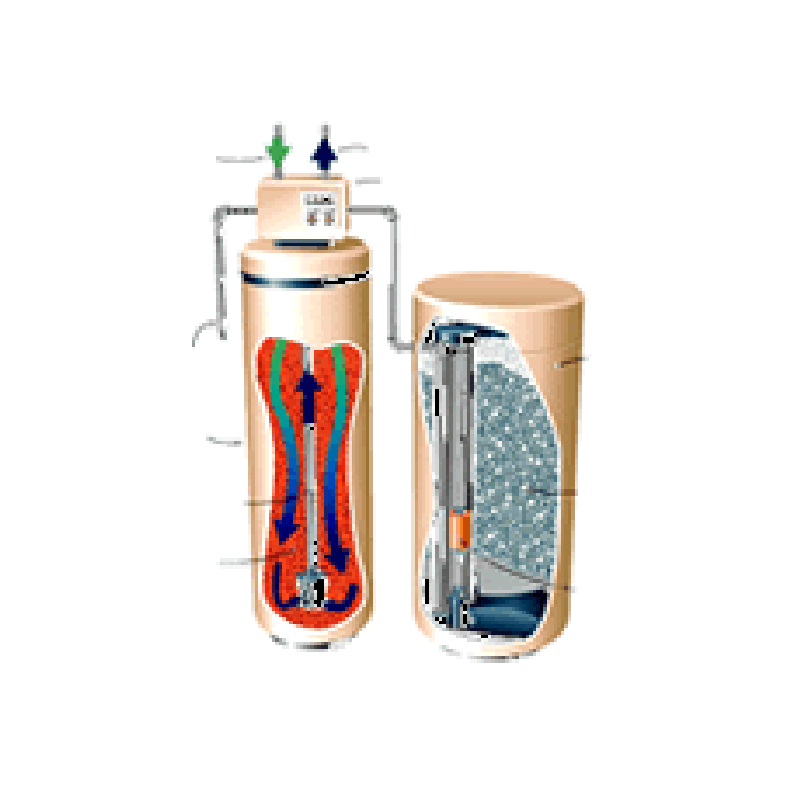
How Do Water Softeners Work?
All water softeners use the same operating principle: They trade the minerals for sodium or potassium chloride, [salt chemicals]. The process is called ion exchange.
The heart of a water softener is a mineral tank. It’s filled with small polystyrene beads, also known as resin or zeolite. The beads carry a negative charge.
In normal operation, hard water moves into the mineral tank and the calcium and magnesium ions move to the beads, replacing salt ions. The salt ions go into the water.
Hard water poses no health hazard. On the other hand, the salt that remains in softened water may be a problem for those on salt- restricted diets. (Ask your doctor)
Disadvantages of a Water Softener
➝ Brine discharge can pollute our water.
➝ Uses salt chemicals.
➝ Reverse osmosis system often used can breed bacteria.
➝ Does not remove chemicals and other contaminants.
➝ Warranty may not cover both parts and labor.
➝ Can waste thousands of gallons of water annually.

The One Water Systems Alternative
Central Water Descaler (CWD-001), provides a way to keep hard water stains and scale buildup out of brand-new systems while progressively getting rid of scale from older, untreated systems. For best outcomes, this descaling process takes several months and happens gradually. Central Water Descaler (CWD-001) doesn't have any negative effects on the quality of drinking water, in contrast to traditional water softeners that use salt. By using this cutting-edge method, it guarantees the durability and effectiveness of water systems without sacrificing the drinking water's quality or safety.
Central Water Descaler (CWD-001) offers a dependable and efficient solution for either reducing or eliminating existing scale deposits or preventing scale formation in recently installed systems. It provides an environmentally friendly substitute for conventional water softening techniques by emphasizing the preservation of system function and water quality With Central Water Descaler (CWD-001), bid hard water problems farewell and welcome to reliably clean, scale-free water.

View calcium bicarbonate (before amp force)

and calcium carbonate crystals (after amp force).

Experience the game-changing feature of the Central Water Descaler (CWD-001) Large Flux Anti-Scaling. Engineered to combat persistent scaling issues in Central Water Descaler (CWD-001), this innovative technology provides a comprehensive solution for maintaining pristine water quality.Scaling, caused by the buildup of minerals like calcium and magnesium, poses a significant threat to the efficiency and longevity of water-based appliances and plumbing systems. Traditional descaling methods often fall short, requiring frequent maintenance and costly repairs. However, with the Large Flux Anti-Scaling feature of the Central Water Descaler (CWD-001), these concerns become a thing of the past.
One Water Systems: Central Water Descaler (CWD-001) - Pure water, redefined in 2024.
How Central Water Descaler Works
Benefits of Central Water Descaler
➝ You will have softer, wetter water to shower in.
➝ You will use less shampoo, conditioner and body wash.
➝ Your skin is going to feel great and your skin won’t itch! Why? Because the softer, wetter water will actually wash the shampoo, conditioner and body wash off your skin, leaving you clean and dry.
➝ No scaling issues with your showerhead!
➝ Glass screens will be cleaner, and if there is any recurring spotting it can be easily wiped away. We still recommend that you still squeegee your shower screen after showering.
➝ Wall and floor tiles will be cleaner and are more easily cleaned…no more scrubbing!
➝ You won’t have to coat yourself in body cream because your skin will already feel great.

